A list of chemical elements is an organized catalog of the known fundamental substances that consist of atoms defined by a specific number of protons in their nuclei. Each element represents a distinct form of matter and is arranged within the periodic table according to atomic number, electron structure, and recurring chemical properties. As of today, 118 elements have been formally recognized, ranging from hydrogen, the lightest, to oganesson, one of the heaviest synthesized in laboratories.
The periodic arrangement provides a framework for understanding chemical behavior, bonding patterns, reactivity, and material properties. It underpins modern chemistry, physics, materials science, and industrial processes, serving as a universal reference for scientific research and technological development.
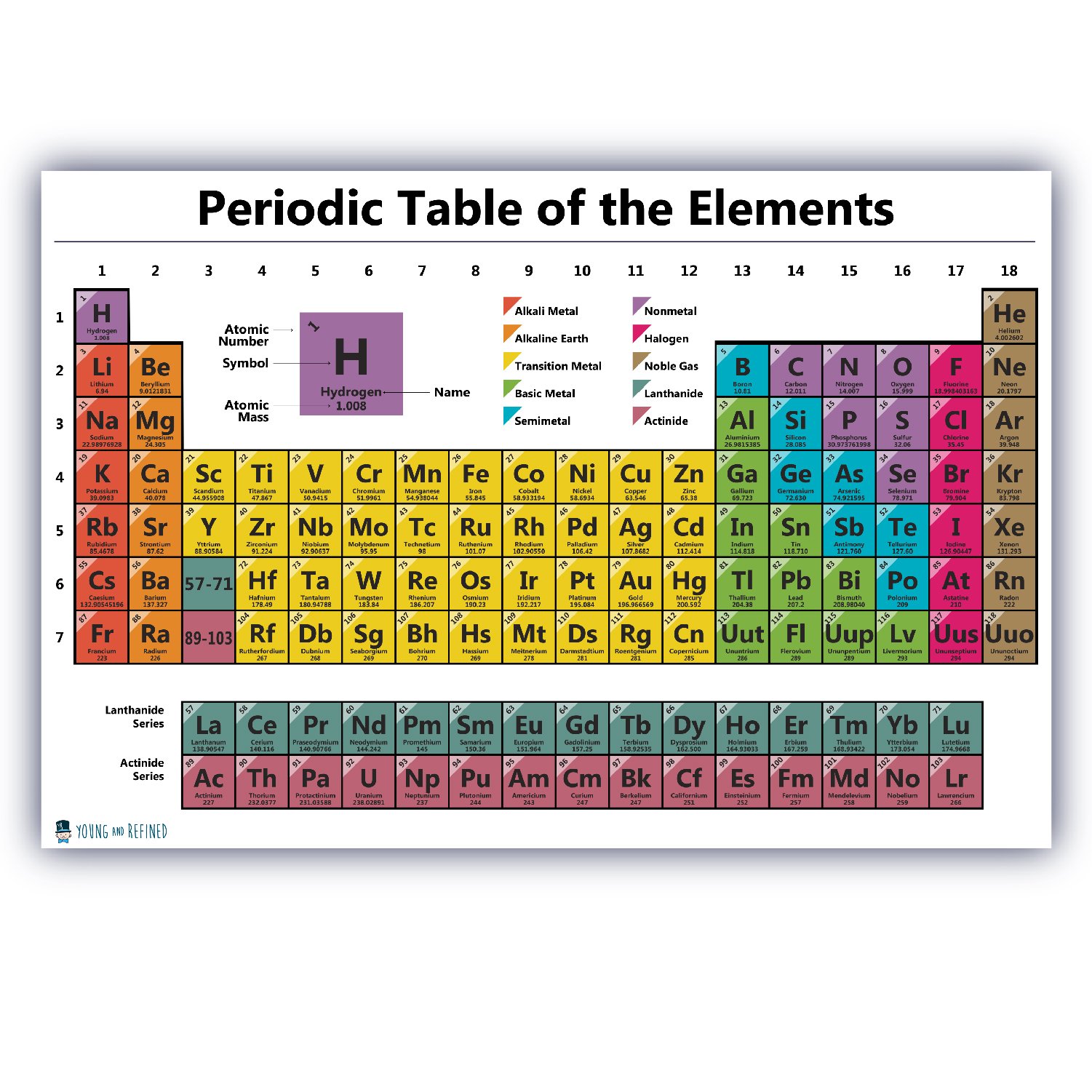
⚛️ Organization of the Periodic Table
Elements are ordered by increasing atomic number and grouped by shared chemical characteristics. Vertical columns, known as groups, contain elements with similar valence electron structures, while horizontal rows, or periods, reflect progressive changes in atomic structure.
This structure allows scientists to predict properties such as reactivity, conductivity, and bonding behavior even for elements that were unknown at the time the table was first proposed.
🧪 Major Categories of Elements
Chemical elements are broadly classified into several functional families based on physical and chemical behavior:
- Alkali metals
- Alkaline earth metals
- Transition metals
- Post-transition metals
- Metalloids
- Nonmetals
- Halogens
- Noble gases
- Lanthanides
- Actinides
These categories reflect differences in electron configuration, ion formation, and bonding patterns.
🔬 Natural vs. Synthetic Elements
Many elements occur naturally in Earth’s crust, oceans, and atmosphere. Others are produced artificially in particle accelerators or nuclear reactors through atomic collisions and radioactive decay processes.
Synthetic elements are typically unstable and exist only briefly before decaying into lighter elements. Their discovery expands understanding of nuclear physics and atomic structure.
📊 Complete List of Chemical Elements (by Atomic Number)
1–20:
Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon,
Sodium, Magnesium, Aluminum, Silicon, Phosphorus, Sulfur, Chlorine, Argon, Potassium, Calcium
21–40:
Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc,
Gallium, Germanium, Arsenic, Selenium, Bromine, Krypton, Rubidium, Strontium, Yttrium, Zirconium
41–60:
Niobium, Molybdenum, Technetium, Ruthenium, Rhodium, Palladium, Silver, Cadmium, Indium, Tin,
Antimony, Tellurium, Iodine, Xenon, Cesium, Barium, Lanthanum, Cerium, Praseodymium, Neodymium
61–80:
Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium,
Lutetium, Hafnium, Tantalum, Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold, Mercury
81–100:
Thallium, Lead, Bismuth, Polonium, Astatine, Radon, Francium, Radium, Actinium, Thorium,
Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium
101–118:
Mendelevium, Nobelium, Lawrencium, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Darmstadtium,
Roentgenium, Copernicium, Nihonium, Flerovium, Moscovium, Livermorium, Tennessine, Oganesson
🌍 Scientific Importance
The catalog of elements forms the foundation for understanding matter and chemical reactions. Every compound, material, and biological system derives from combinations of these elemental building blocks.
The table also reflects ongoing research in nuclear chemistry and particle physics, where scientists continue to investigate the limits of atomic stability and the possibility of discovering new elements beyond the current range.
Last Updated on 16 hours ago by pinc